CEP Program
(Chemotherapy Enhancement Program)
Veyonda® combined with chemotherapy
Phase-1 multi-centre study – complete
CEP-1 was a two-part study:
Of the 9 patients allocated to the higher dose (800 mg) of Veyonda, 5/9 (56%) showed stable disease (no significant tumour growth and no new tumours) or a partial response (significant shrinkage of measurable lesions) over the 8-month term of the study.
Findings from this study have been published in the independent peer-reviewed Journal Current Therapeutic Research in 2021.[i]
Phase-1 multi-centre study - closed
CEP-2 was a Phase 1, open-label, study of Veyonda administered to cohorts of patients being treated with doxorubicin for the treatment of metastatic soft tissue sarcoma.
Patients in the United States with a range of soft tissue sarcomas were enrolled across six US sites to be treated with the Veyonda / doxorubicin combination as a first-line treatment.
Soft tissue sarcomas are generally very aggressive cancers. Up to 50% of high-grade sarcoma patients develop metastases and die within 12 months. They are defined as a rare cancer, with fewer than 20,000 new cases diagnosed in the US in 2021.
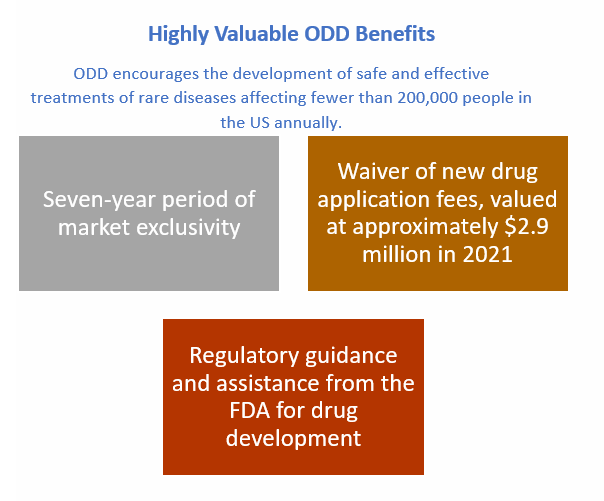
Veyonda was granted US FDA Orphan Drug Designation (ODD) for the treatment of sarcoma. This confers a period of market exclusivity and a number of financial and regulatory benefits.

ODD confers the following benefits:
The CEP-2 Safety Steering Committee found that a dose of Veyonda up to 1200mg was safe.
The study is now closed.
[i] Kiknavelidze K, Shavdia M, Chikhladze N, Abshilava L, Messina M, Mautner G, Kelly G. NOX66 as Monotherapy, and in Combination With Carboplatin, in Patients With Refractory Solid Tumors: Phase Ia/b Study. Curr Ther Res Clin Exp. 2021 Mar 28;94:100631. DOI: 10.1016/j.curtheres.2021.100631