Autoimmune Disease
Autoimmune diseases including lupus, psoriasis, type 1 diabetes, rheumatoid arthritis and multiple sclerosis affect millions of people worldwide.
These conditions are characterised by uncontrolled inflammation that contributes to many chronic health issues and has an enormous impact on longevity and quality of life.
It is a sector with significant commercial potential. The global market for autoimmune disease therapeutics is anticipated to reach US$185 billion by 2029, growing at a CAGR of 3.7%.
Noxopharm’s Sofra™ technology platform is addressing the autoimmune market with SOF-SKN™, its first Sofra drug candidate, moving to clinical trial in 2025.
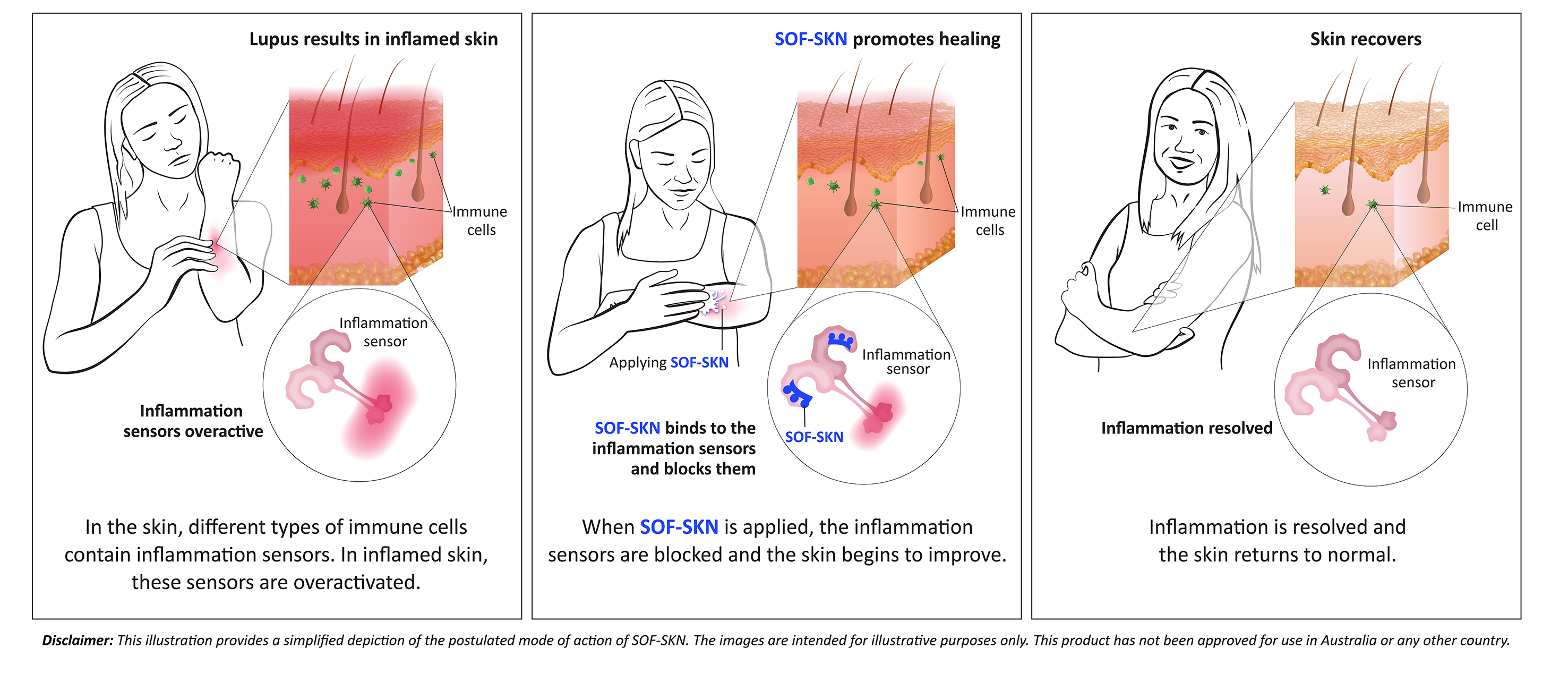
SOF-SKN is a topical treatment that targets an inflammatory sensor called Toll-like receptor 7 (TLR7) using a novel strategy originally developed by Hudson Institute of Medical Research and now in-licensed to the company.
After years of study, researchers have identified a direct link between overactivation of TLR7 and lupus, validating the importance of this target and the focus of the clinical trial.
SOF-SKN contains a proprietary ultra-short oligonucleotide that is used to block TLR7, which is overactivated in autoimmune diseases.
The drug therefore has the potential to change the treatment paradigm of the skin disease caused by cutaneous lupus erythematosus (CLE) from merely controlling symptoms to actually treating the disease itself right at the source.
Click on the image below to view a video demonstrating SOF-SKN’s mechanism of action.

SOF-SKN preclinical development work has been presented to international experts at major medical conferences, among them the 15th International Congress on Systemic Lupus Erythematosus.
Download the Sofra & SOF-SKN Autoimmune and Inflammatory Fact Sheet pdf here
SOF-SKN is not approved for use in Australia or any other country.


